
Question 1.17: Which of the following lattices has the highest packing efficiency (i) simple cubic (ii) body−centred cubic and (iii) hexagonal close−packed lattice? Chapter 1: the Solid State Chemistry Class 12 solutions are developed for assisting understudies with working on their score and increase knowledge of the subjects. Which of the following lattices has the highest packing efficiency, simple cubic, body-centred cubic, hexagonal close-packed lattice is solved by our expert teachers. You can get ncert solutions and notes for class 12 chapter 1 absolutely free. NCERT Solutions for class 12 Chemistry Chapter 1: the Solid State is very essencial for getting good marks in CBSE Board examinations
Question 1.17: Which of the following lattices has the highest packing efficiency (i) simple cubic (ii) body−centred cubic and (iii) hexagonal close−packed lattice?
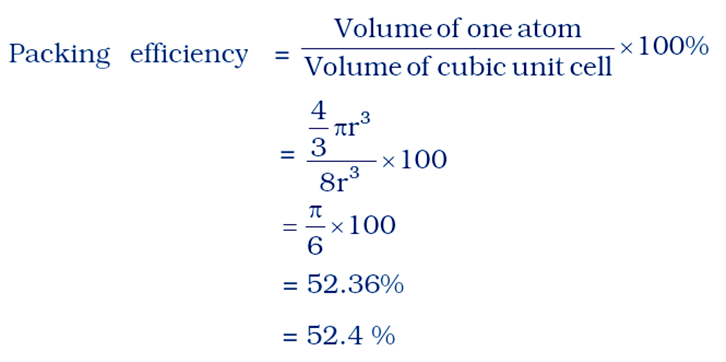
Answer a = 2r
The volume of the cubic unit cell = side3
= a3
= (2r)3
= 8r3
Number of atoms in unit cell = 8 × 1 /8
= 1
The volume of the occupied space = (4/3)πr3
(ii) In body centered cubic two atoms diagonally
Let take edge length or side of the cube = a,
Let take radius of each particles = r
The diagonal of a cube is always a√3
The relation between radius and edge a will
a√3 = 4r
divide by root 3 we get
a = 4r/√3
total number of atoms in body centered cubic
number of atoms at the corner = 8 × 1/8 = 1
number of atoms at the center = 1
total number of atoms = 2
The volume of the cubic unit cell = side3
= a3
= (4r/√3)3
The volume of the occupied space = (4/3)πr3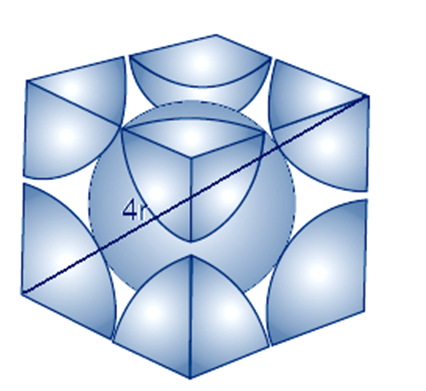
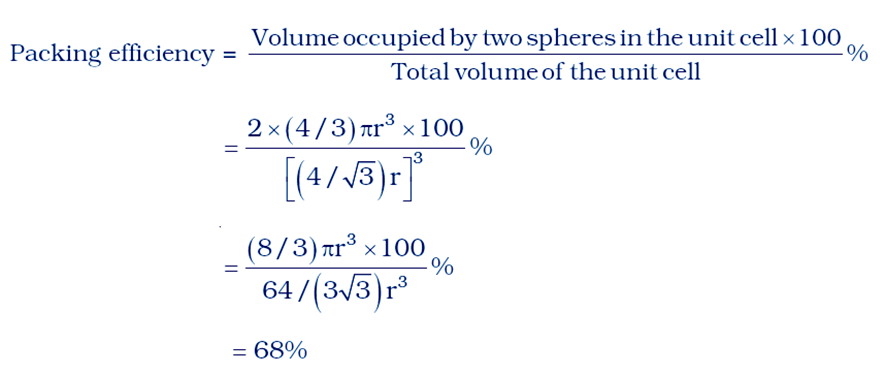
(iii) hexagonal close−packed lattice
Let take base of hexagonal is a and height is c
Each angle in hexagonal will 60 degree at base 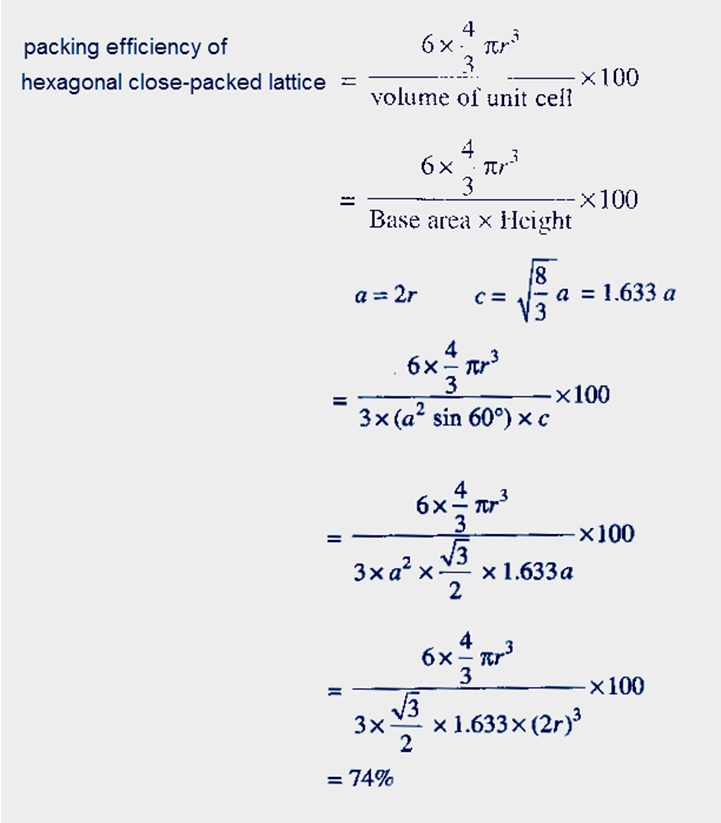
Hexagonal close−packed lattice has the highest packing efficiency of 74%.
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.